Sontra's continous non-invasive glucose monitor, the Symphony™ Diabetes Management System, is being co-developed with Bayer Diagnostics. Their glucose monitor measures glucose diffusing through ultrasonically permeated skin continously for up to twenty four hours. The product consists of SonoPrep instrument, a glucose biosensor patch with RF transmitter and a glucose meter . For the home use monitor, the glucose biosensor will be worn under clothing to reduce lifestyle constraints. The PDA/ glucose meter will provide on demand blood glucose readings in addition to trends and alarms that will minimize the frequency of high and low blood glucose levels.
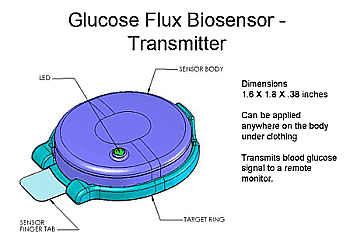
In the fall of 2004, Sontra completed its second human clinical study at Vanderbuilt University that demonstrated the Symphony reliably detected real-time changes in blood glucose levels. Symphony had a 90% correlation (r = 0.9) to reference blood glucose and reliably detected hypoglycemia. Product development is currently focused on improvements to the glucose flux biosensor and design of the wireless RF interface between the biosensor and glucose meter. Sontra expects commence FDA clinical studies in 2006 for continuously monitoring the glucose of surgical patients in the intensive care unit (ICU). In parallel, Sontra is developing a home-use product for patients with diabetes.
