People Choose CGMs For:
- Better Glucose Management
- Avoiding frequent hypoglycemia
- Preventing hypoglycemia unawareness
- Understanding the effects of particular foods on after-meal glucose levels
- Overnight glucose safety
- Living alone
- Frequent driving, travel, high-risk professions
- A young child unable to report hypoglycemia
- Tight control before and during pregnancy
- Avoiding diabetes complications
A continuous glucose monitor reveals short-term trends in blood sugar as they happen. The monitor reads out a constant stream of glucose data every 1 to 5 minutes. You can see the direction your blood sugar is taking in the last 1, 3, 6, 9, 12, or 24 hours, depending on what times your specific monitor offers. This gives 288 to 1440 readings daily compared to the 4 to 8 fingerstick readings you rely on with a meter.
A CGM acts like a diabetes coach who shows you how boluses, exercise, carb intake, and treatment of lows and highs affect glucose readings. Once undesirable patterns are identified, you can work toward preventing them from insulin and lifestyle adjustments.
Parts of a Continuous Glucose Monitoring System
CGMs have three essential components: a sensor, a transmitter, and a receiver. The receiver and transmitter are durable devices that are reused each time the sensor is changed. Sensors are disposable and approved for 3 to 14 days of use, depending on the manufacturer. In practice, sensor life varies from person to person, and sensors can sometimes be used beyond their stated life. The main concern with more prolonged use is an infection, but the risk of this appears to be low compared to infusion sets. At some point during extended use, readings will become less accurate. When inaccurate readings occur, you can try calibrating the sensor several times over an hour or so but don’t continue to use it if readings remain inaccurate. Be sure to discuss this with your physician. Irritation at the site from the sensor or tape is unusual, but check for this upon removal.
CGM Site Tips
- Approved for the abdominal area, many people wear their CGM on the back of the arms, outer thighs, upper buttocks, or love handles.
- Avoid scar tissue and shave areas where hair may prevent good adhesion.
- Pinch up the skin during insertion to prevent the insertion needle from going too deep, BUT make sure it goes deep enough to get into fat tissue.
- If you use an adhesive barrier under the sensor, such as IV 3000, cut a hole where the sensor goes through.
- If you use Skin Tac or IV Prep pads to improve adhesion, avoid gumming up the skin near the point where the sensor will go into the skin.
Sensor
A sensor is a thin, flexible wire inserted beneath the skin with a needle and an insertion device. The sensor rests in the fatty layer below the skin and is usually not felt by the person wearing it. Sensor wires vary from 1/4 to 3/5 inches (6 to 15 mm) in length. Current introducer needles vary from less than 26 gauge to 22 gauge. Sensors contain enzymes that react with glucose in the interstitial fluid around cells. A chemical reaction produces an electric signal that the transmitter sends to the CGM receiver, which is interpreted as a glucose level.
Any site at least two or three inches away from the last sensor site and the infusion set can be used. Choose any fleshy site without scars or bruises. Swab the site with alcohol and insert the sensor (Pump Preparation). Some sensors can also be inserted by hand. Once the sensor is in place, the inserter and needle are removed. Unlike pump infusion sites, infections are rare with sensor sites because nothing is infused into the body. Bleeding can occur, so check the site for bleeding and wipe it clean if needed. If significant bleeding occurs, use another sensor on a different site. Check periodically for redness, tenderness, or swelling.
Transmitter
Once the sensor is inserted, a reusable waterproof transmitter is seated into a plastic pod on top of the sensor or clicked into the side of the sensor. Transmitters vary in size and shape from 1/4 to ½ inch (6 to 12 mm) thick and from a thumbnail to a half dollar in circumference.
Transmitters vary in how far they can send readings, currently about 5 to 10 feet (2 to 3 meters). Cell phones and other electronic devices can occasionally interfere with data transmission.
Receiver
A battery-powered receiver or a display on the pump or pump controller displays glucose information received from the transmitter. Separate receivers are about the size of a small cell phone and can be carried in a pocket, backpack, or purse or worn on a belt. With pump receivers, the user does not have a separate receiver.
Current U.S. insulin pumps don’t use CGM readings to adjust insulin delivery, but clinical trials are underway that will gradually add more closed-loop features to pumps. Some trials are trying to close the loop with the wearer giving an approximate bolus for a meal to reduce post-meal glucose spiking. This allows slower bolus insulin to get started before the meal, with the CGM and pump handling the smaller dose adjustments that would then be required.
The receiver displays glucose values every five minutes, shows trend graphs of past glucose values over the last several hours (1 to 24 hours), and has arrows and trend lines that show whether the glucose is rising or falling.
Various companies have already released continuous monitors, with more companies developing theirs every day. Significant differences in accuracy can be seen in one individual when two different continuous monitors are worn at the same time.
Available Monitors (See Comparison):
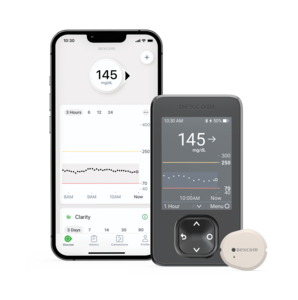
 Dexcom G6 and G7
Dexcom G6 and G7
- Manufacturer: Dexcom
- FDA Approval: Dexcom G6 was approved in 2018; Dexcom G7 received FDA approval in December 2022.
- EU Approval: Both systems have CE Mark approval for use in the European Union.
- Features: Real-time glucose monitoring with readings every five minutes; the G7 offers a smaller, all-in-one wearable design with a 30-minute warm-up period.
FreeStyle Libre Systems (Libre 2 and Libre 3)
- Manufacturer: Abbott Laboratories
- FDA Approval: FreeStyle Libre 2 was approved in 2020; FreeStyle Libre 3 received FDA approval in May 2022.
- EU Approval: Both systems have CE Mark approval.
- Features: Continuous glucose monitoring with optional alarms for high and low glucose levels; Libre 3 offers real-time readings every minute and a smaller sensor size.
 Eversense E3 and Eversense 365
Eversense E3 and Eversense 365
- Manufacturer: Senseonics
- FDA Approval: Eversense E3 was approved in February 2022; Eversense 365 received FDA approval in September 2024.
- EU Approval: Both systems have CE Mark approval.
- Features: Implantable CGM systems with long-term use; E3 lasts up to 180 days, while Eversense 365 is designed for up to one year of continuous monitoring.
Medtronic Guardian Connect and Simplera
- Manufacturer: Medtronic
- FDA Approval: Guardian Connect was approved in 2018; Simplera is currently under review for FDA approval.
- EU Approval: Both systems have CE Mark approval.
- Features: Guardian Connect offers real-time glucose monitoring with predictive alerts; Simplera is a simplified CGM sensor aimed at enhancing user experience.
Dexcom Stelo Glucose Biosensor System
- Manufacturer: Dexcom
- FDA Approval: Approved in March 2024 as the first over-the-counter (OTC) continuous glucose monitor for individuals aged 18 and older who do not use insulin.
- Features: Designed for non-insulin users, including those with type 2 diabetes or individuals seeking to monitor glucose levels for lifestyle reasons.
 Accu-Chek SmartGuide
Accu-Chek SmartGuide
- Manufacturer: Roche
- EU Approval: Received CE Mark approval in July 2024.
- Features: AI-enhanced CGM capable of predicting the risk of hypoglycemia from 30 minutes to seven hours ahead; sensor can be worn for up to 14 days.
These CGM systems offer a range of features tailored to meet diverse needs, from long-term implantable sensors to over-the-counter options for non-insulin users. It’s essential to consult with a healthcare professional to determine the most suitable CGM system based on individual health requirements and lifestyle.

 Dexcom G6 and G7
Dexcom G6 and G7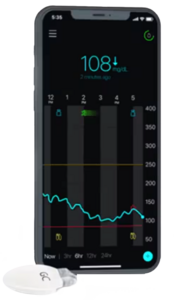 Eversense E3 and Eversense 365
Eversense E3 and Eversense 365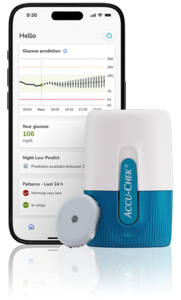 Accu-Chek SmartGuide
Accu-Chek SmartGuide